Accu-Chek Solo Tubeless Patch Pump
Tubeless Patch Pump, For Extra Possibilities.
Tubeless, small & lightweight; so you hardly know it's there.
Accu-Chek Solo Tubeless Patch Pump
Tubeless Patch Pump, For Extra Possibilities.
Tubeless, small & lightweight; so you hardly know it's there.
Features & Benefits

- Tube-free and small Tube-free and small for more freedom
- Detachable Take it off and put it on a twist
- Open bolus adviser To combine therapy with CGM
- Quick bolus buttons Quick bolus buttons on pump for extra piece of mind
- Modular design To change only parts instead of the whole pump

Technical Specifications
Refer to the package insert that came with your meter for the latest information on device specifications and product restrictions.
Micropump System
| Permitted insulin types | U100 insulins: Humalog®, NovoLog®, NovoRapid®, Apidra®, Insuman® Infusat, Fiasp® |
| Electromagnetic compatibility | The micropump system meets the EMC requirements for home healthcare environments in accordance with IEC 60601-1-2. |
| Electromagnetic emission | Classified in accordance with CISPR 11, group 1, class B (residential). |
| Safety | The safety concept is based on a control system that consists of two microprocessors and a supervisor microprocessor (supervising system). The control system has a dual channel software architecture that performs all safety-relevant functions twice. Whenever a defect or fault occurs in the control system, it is identified by the supervisor microprocessor and vice versa. The control and supervising systems signalise errors by means of acoustic signals and messages on the diabetes manager screen. |
| Communication between micropump and diabetes manager | Bluetooth Low Energy (BLE) wireless technology |
| Transmission frequency | 2,402–2,480 MHz |
| Transmission power | 1 mW / 0 dBm Channels: 37*FHSS + 3*DSSS advertising channels Modulation: GFSK Bandwidth: 1 MHz “single hop frequency” |
| Communication range | 2 m (the range may be impaired by obstacles) |
Diabetes Manager
| Device type | Accu-Chek Guide Solo diabetes manager The Accu-Chek Guide Solo diabetes manager is suitable for continuous operation. |
| Expected Service Life | 4 years |
| Access control | PIN-based protection |
| Dimensions | 124 × 64 × 17 mm (L × W × H) |
| Weight | 140 g |
| Signal reproduction | Graphical user interface, status LED, speakers, vibration alarm |
| Screen | Capacitive colour LCD multi-touch screen with backlight |
| Screen size | 3.5" |
| Screen resolution | 320 × 480 pixels |
| Screen timeout | After 2 minutes of no activity |
| Camera | 2 megapixels for scanning the pairing code (2D data matrix code) at a minimum of 300 lx up to a maximum of 20,000 lx |
| Admissible temperature range | Storage and transport, with packaging: -20 °C to +50 °C During operation: +5 °C to +40 °C Storage between periods of use: -25 °C to +70 °C Cooling-down time from maximum storage temperature between periods of use to operating temperature, at an ambient temperature of 20 °C: 15 minutes 1 Warming-up time from minimum storage temperature between periods of use to operating temperature, at an ambient temperature of 20 °C: 15 minutes |
| Admissible humidity range | Storage and transport, with packaging: 5 % to 85 % During operation: 15 % to 90 % |
| Atmospheric pressure | Storage and transport, with packaging: 54.9 kPa to 106 kPa (549 mbar to 1,060 mbar) During operation: 70 kPa to 106 kPa (700 mbar to 1,060 mbar) During charging: 80 kPa to 106 kPa (800 mbar to 1,060 mbar) Storage between periods of use: 54.9 kPa to 106 kPa (549 mbar to 1,060 mbar) |
| Operating height | Up to 3,000 m above sea level (diabetes manager) Up to 2,000 m above sea level (charger) |
| Signal types | Visual, acoustic, vibration |
| Sound pressure level of the signal | ≥ 45 dBA at a distance of 1 m |
| Frequency of the signals | 1–3 kHz |
| Interface to PC | USB 2.0 (micro-B) |
| Memory capacity | 5,000 blood glucose tests, 5,000 logbook entries, 5,000 pump events |
| Power supply | Rechargeable lithium polymer battery Model: Nugen |
| Battery voltage | 3.7 V |
| Battery capacity | 1,530 mAh |
| Charging voltage via USB | 5 V |
| Max. charging current | 700 mA |
| Charging voltage via USB | 5 V |
| USB charger | Technics switch-mode power supply, model TS051X110-0502R |
| IP rating | IP20 |
| Bolus calculator | Accu-Chek Bolus Advisor |
| Test strip slot | Illuminated test strip slot for Accu-Chek Guide test strips |
| Measuring range | 0.6–33.3 mmol/L |
Micropump
| Dimensions | Approx. 63 × 39 × 14 mm |
| Weight | Micropump with filled reservoir < 29 g |
| Pump casing | Impact and scratch-resistant plastic (polycarbonate) |
| Quick bolus buttons | Silicone buttons for delivering quick boluses, turning flight mode on/off and muting messages temporarily |
| Admissible temperature range | Storage and transport, with packaging (pump base): -20 °C to +50 °C Storage and transport, with packaging (reservoir): +10 °C to +30 °C During operation and storage between uses: +5 °C to +40 °C Cooling-down time from maximum storage temperature between periods of use to operating temperature, at an ambient temperature of 20 °C: 10 minutes Warming-up time from minimum storage temperature between periods of use to operating temperature, at an ambient temperature of 20 °C: 10 minutes |
| Admissible humidity range | Storage and transport, with packaging (pump base): 5 % to 85 % Storage and transport, with packaging (reservoir): 20 % to 80 % During operation and storage between uses: 15 % to 90 % |
| Atmospheric pressure | Storage and transport, with packaging: 54.9 kPa to 106 kPa (549 mbar to 1,060 mbar) During operation: 70 kPa to 106 kPa (700 mbar to 1,060 mbar) Storage between periods of use: 54.9 kPa to 106 kPa (549 mbar to 1,060 mbar) |
| Motor type | Stepper motor |
| Power supply | 1.4 V zinc-air battery for internal power supply |
| Life expectancy of the battery | If used in a typical usage pattern (50 U/day using U100 insulin; room temperature: 23 °C ±2 °C), life expectancy of the battery is up to 4 days. |
| Basal rate | Minimum: 0.1 U/h Maximum: 25.0 U/h |
| Basal rate delivery accuracy | ±16 % or better at 0.1 U/h ±5 % or better at 1.0 U/h |
| Basal rate, increments | 0.1 U/h up to under 5.0 U/h: increments of 0.01 U/h 5.0 U/h up to under 25.0 U/h: increments of 0.1 U/h |
| Basal rate profiles | Up to 5 customised profiles |
| Temporary Basal Rate (TBR) | 0–90 % for basal rate reductions and 110–250 % for basal rate increases in increments of 10 % The duration is adjustable in 15-minute increments for a time period of up to 24 hours. Up to 5 individual TBRs can be programmed |
| Bolus types | Standard bolus, quick bolus, extended bolus, multiwave bolus |
| Bolus amount | Minimum: 0.2 U Maximum: 50 U |
| Bolus delivery accuracy | ±30 % or better at 0.2 U ±5 % or better at 50.0 U |
| Bolus amount, increments | 0.2 U up to under 2.0 U: increments of 0.05 U 2.0 U up to under 5.0 U: increments of 0.1 U 5.0 U up to under 10.0 U: increments of 0.2 U 10.0 U up to under 20.0 U: increments of 0.5 U 20.0 U up to 50.0 U: increments of 1.0 U The duration of an extended bolus or a multiwave bolus is adjustable in 15-minute increments for a time period of up to 24 hours. |
| Delivery lag time | Adjustable in 15-minute increments from 0 to 60 minutes |
| Quick bolus increment | 0.2 U / 0.5 U / 1.0 U and 2.0 U |
| Delivery speed | Boluses: 1.0–2.5 U/min. Filling the reservoir needle: 1.0–2.5 U/min. |
| Sound pressure level of the signal | ≥ 45 dBA at a distance of 1 m |
| Occlusion detection | Rotation detector |
| Maximum amount of time until occlusion message M-24 | 50 hours at a basal rate of 0.1 U/h 5 hours at a basal rate of 1 U/h |
| Maximum insulin amount until occlusion message M-24 | 5.0 U |
| Maximum pressure | 150 kPa (1.5 bar) |
| Reservoir fill amount | Maximum: 200 U Minimum: 80 U |
| Maximum overdelivery in the event of an error | 0.4 U |
| IP rating | IP22 |
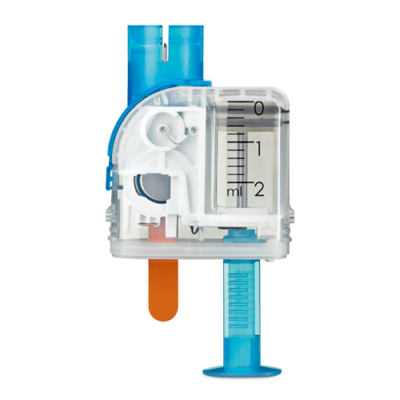
Infusion Assembly
| Pump holder | Dimensions: Approx. 67 × 32 × 6.5 mm |
| Adhesive pad | Dimensions: Approx. 85 × 52 mm |
| Cannula | Orange: 6 mm, soft Teflon® catheter, 90° insertion angle Blue: 9 mm, soft Teflon® catheter, 90° insertion angle |
| Cannula fill amount | 0.7 U |
| Maximum period of use | up to 3 days |
| Sterility | Sterilised using ethylene oxide for single use according to EN ISO 11135 |
Insertion Device
| Dimensions | 82 × 53 × 49 mm |
| Weight | 85 g |
| Period of use | Approx. 1 year You can program a reminder in the diabetes manager to remind you to replace the insertion device before the end of the period of use. |
Related Products
Discover the supporting products you need to optimize your Insulin Delivery System